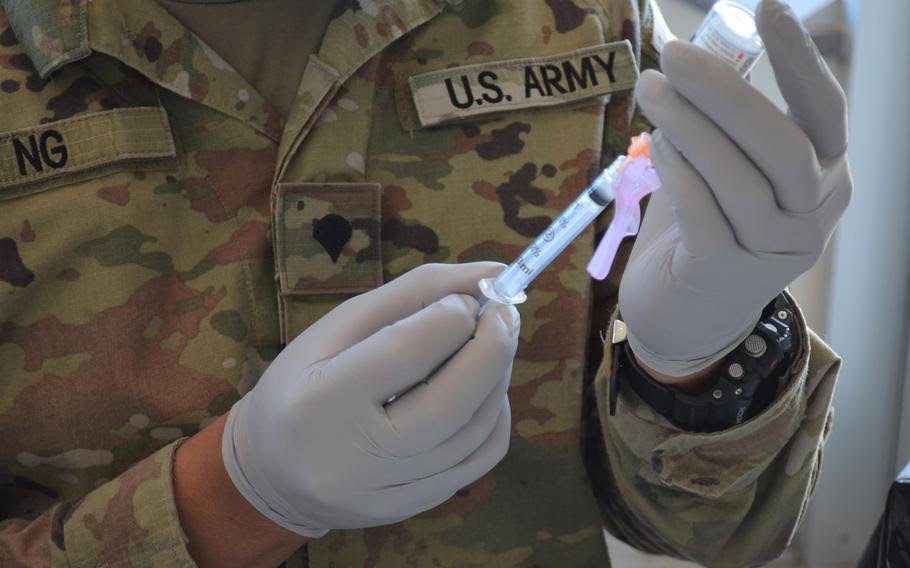
National Guard ships recently approved coronavirus vaccine to units
A coronavirus vaccine is prepared for injection at Fort Hood, Texas,
The National Guard began shipping the newly approved Novavax coronavirus vaccine this month to its units across the country in an effort to appeal to those service members resisting previously released vaccines.
The National Guard Bureau said Thursday that the vaccines, which are based on more traditional technology, should arrive at units this month. It will take about 90 days to get reportable data on troops’ acceptance of the shot, bureau officials said.
It is more than a month past the Guard’s June 30 deadline to be immunized against the coronavirus and about 10% of the 336,000 Army National Guard troops, or approximately 33,600 members, have not received at least one vaccine shot. In the total Guard force of 440,000 soldiers and airmen, about 39,600 remain unvaccinated, according to the National Guard Bureau.
Unvaccinated Guard troops have been cut off from some of their benefits and barred from participating in federal training, drills and other military duties. The Guard has not announced any separations that have resulted from refusing the vaccine.
Novavax uses a two-dose regimen, which is similar to the more popular vaccines made by Pfizer and Moderna, though the way it builds immunity in the body is different. Novavax uses proteins to deliver the virus, while the other two vaccines use a new technology known as mRNA.
Many seeking religious exemptions from the vaccines have done so because mRNA vaccine technology was developed or tested with fetal cell lines derived from elective abortions that occurred in the 1970s and 1980s, according to UCLA Health. However, none of the vaccines contain aborted fetal cells.
The Navy and Air Force also have begun making the Novavax vaccine available to service members after it was approved last month for emergency authorization for adults and the national Centers for Disease Control and Prevention endorsed it.
“Most airmen and guardians have already received vaccines using similar technology as the Novavax COVID-19 vaccine, like the hepatitis B vaccine, which is a Department of Defense requirement. Other vaccines produced with similar technology are the human papillomavirus vaccine and even one of the flu vaccines,” Lt. Col. David Sayers, chief of preventive medicine for the Air Force Medical Readiness Agency, said in a statement. “The Novavax COVID-19 vaccine uses technology that has been around since the 1980s. Not only do we have effectiveness and safety data from the Novavax clinical trials, but we also have decades of experience with this type of vaccine.”
In clinical trials performed before the emergence of the delta and omicron variants of the virus, Novavax was 90.4% effective in preventing mild, moderate or severe coronavirus infections, according to the Air Force. Unvaccinated service members must receive the two doses — taken at least 21 days apart — to be considered fully vaccinated and meet the Defense Department vaccine requirement.